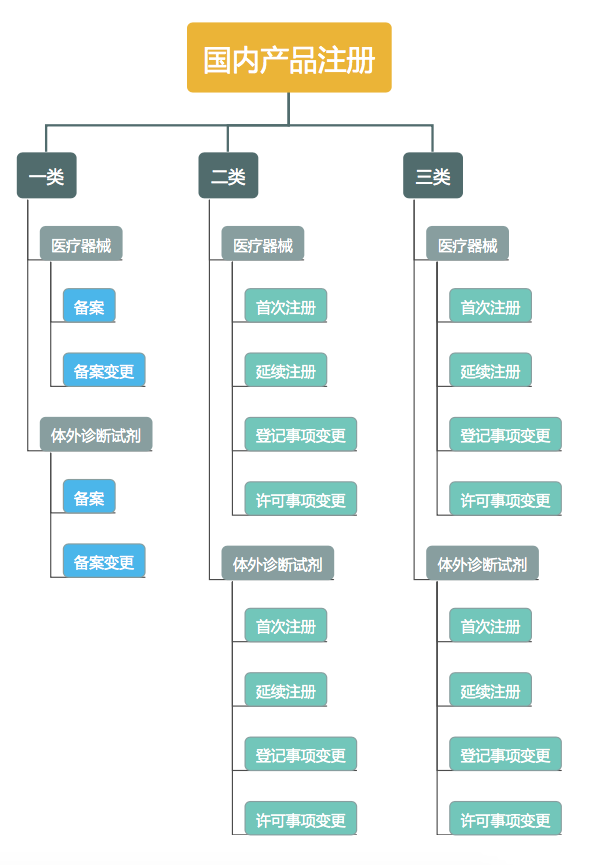
To register for the first time
It is the first time for the products to be registered in China Food and Drug Administration (NMPA). The issued certificate is valid for five years.
Continue to register
Application for renewal of product certificate validity 6 months prior to its expiry. Each application is renewed for five years.
Registration of change
For registered medical devices of Category II and Iii, if the contents contained in the medical device registration certificate and its attachments change, the registrant shall apply for registration change to the original registration department. Registered changes are divided into changes in registered items and changes in permitted items.
Change of Registered items
Where the name and domicile of the registrant or the name and domicile of the agent are changed, the registrant shall apply to the original registration department for changing the registered items; Where the address of domestic medical device production is changed, the registrant shall change the registered items after the corresponding production license is changed.
Change of license
Where the product name, model, specification, structure and composition, scope of application, product technical requirements, and manufacturing address of imported medical device are changed, the registrant shall apply to the original registration department for permission to change the items.
For the record
Before the production of category I medical devices, the registration of the products shall be handled. There is no validity limit.
For the record the change
If the contents and technical requirements of the archival information form for category I medical devices are changed, the archival person shall propose the alteration of the archival information to the original archival department.