Unique Device Identification (UDI) is a code composed of numbers, letters or symbols attached to medical Device products or packages for Unique Identification of medical devices.
The complete UDI system consists of three parts: unique identifier, data carrier and database. Electronic ID cards with unique identification for medical devices; Data carrier is the data medium that stores or transmits the unique identification of medical device. A database is a database that stores product identification and associated information with unique identification of medical devices.
In the information age, UDI is a key basic element to realize automatic identification, precise recall, tracing, whole-process general inspection, information interconnection and intelligent management of medical device products.
What does a UDI consist of?
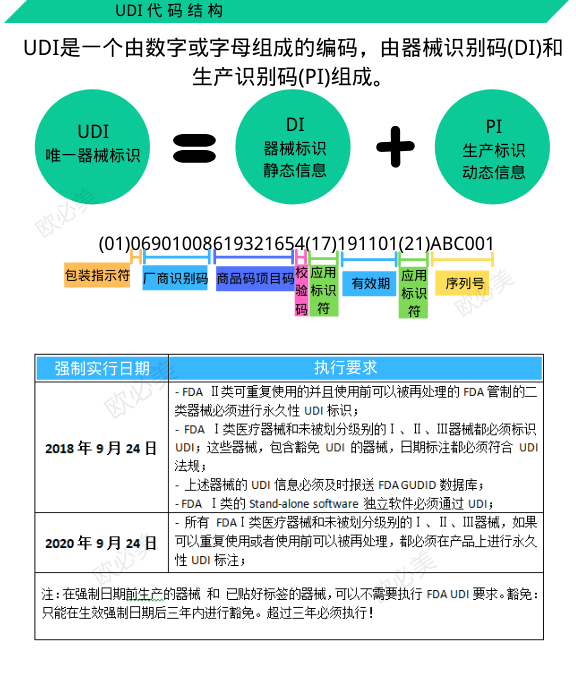
UDI consists of Device Identification (DI) and/or Production Identification (PI).
DI is a mandatory and fixed part of a UDI. It consists of an enterprise identification code and a product specification code. The enterprise identification code is applied for by the registrant or the record holder, and accepted by the issuing institution in accordance with China's medical device coding rules and coding standards, and assigned the unique enterprise identification code in the world. Product specification code refers to the code compiled by the registrant or the filing person according to the characteristics of the product model, specification and packaging. The DI, consisting of an enterprise ID and a product specification code, is unique in the world.
PI is an optional and variable part of the unique identification of a medical device, including production date, expiration date, batch number or serial number, etc. PI data does not need to be uploaded to the coding database, and all units can use universal scanning equipment to locally parse relevant production information.
UDI should conform to the principles of uniqueness, stability, and extensibility. Uniqueness is the primary principle of UDI, ensuring that the unique identification of products is not repeated is the basis of accurate identification, is also the core principle of unique identification function; The principle of stability requires that UDI should be related to the basic characteristics of the product. If the basic characteristics of the product remain unchanged, the product identification should remain unchanged. The extensibility principle requires that UDI should adapt to evolving regulatory requirements and practical applications.