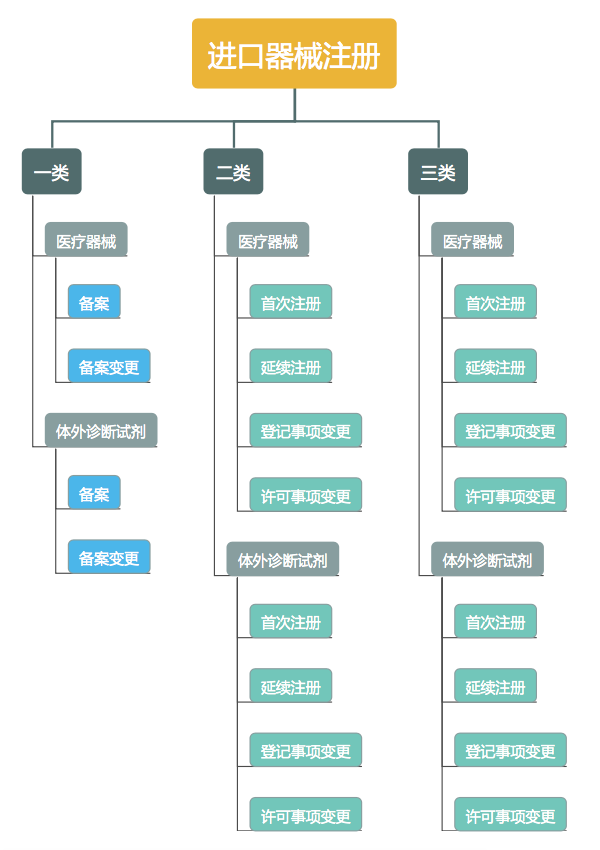
The product registration service items for imported medical devices are the same as those for domestic products.
I. Application Form
2. Supporting documents
1. Certification documents of the country of origin allowing the product to be marketed and sold.
2. Production enterprise qualification documents.
3. A copy of the power of attorney, the letter of commitment of the agent and a copy of the business license or the registration certificate of the agency.
List of basic requirements for safety and effectiveness of medical devices
4. Review data
5. Research data
6. Manufacturing information
7. Clinical evaluation data
8. Product risk analysis data
9. Product technical requirements
10. Product inspection report
Product specification and label sample of minimum sales unit
Declaration of conformity