• Applicant information -- applicant's name, address, etc
• Product description - Product name, grade, shape and structure, purpose of use and other product information
• Labeling, information about sterilization
• Perfomance Standard: Describes the performance standards applicable to the product
• Material evidence of substantial equality
• Test inspection report: data on test inspection results of performance and bioconformance
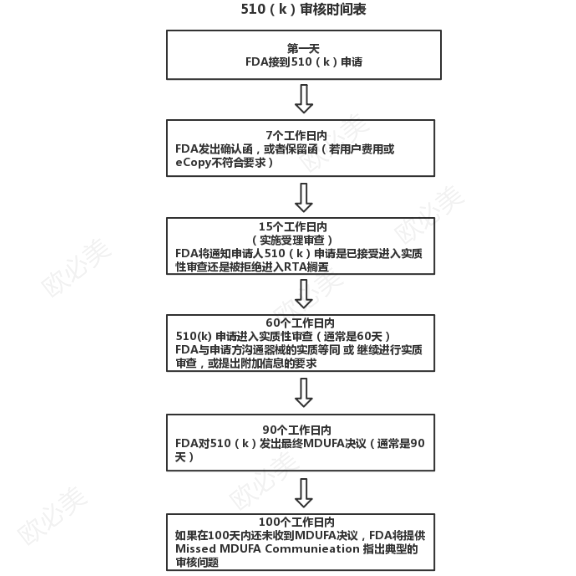
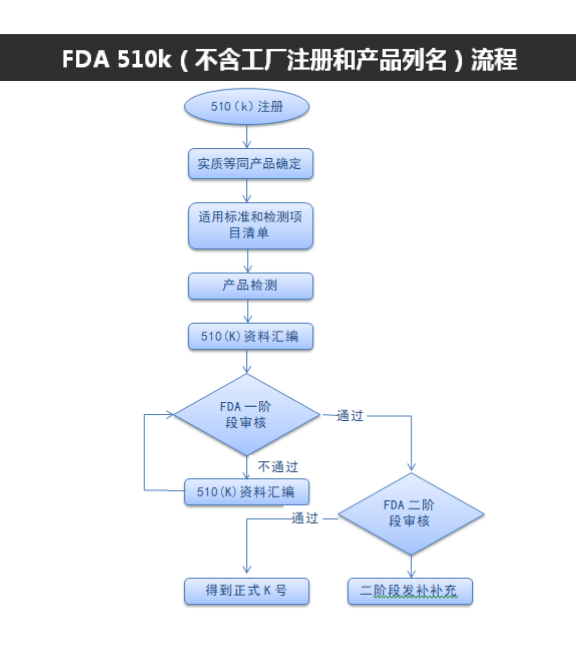
510(K) Premarket license content
PMA requires additional information for safety and effectiveness audits in addition to general matters, and additional GMP on-site audits for final approval
• Preclinical Laboratory Testing Data
- Tests on physical, chemical and mechanical properties of instruments and materials
- Biological conformance tests such as toxicity tests for materials
• Animal Testing Data
- New materials are suitable for deformed materials, artificial pacemakers, etc
• Human Clinical Testing Data